US company trials coronavirus vaccine candidate in Australia
A U.S. biotechnology company began injecting a coronavirus vaccine candidate into people in Australia on Tuesday with hopes of releasing a proven vaccine this year, AP reported.
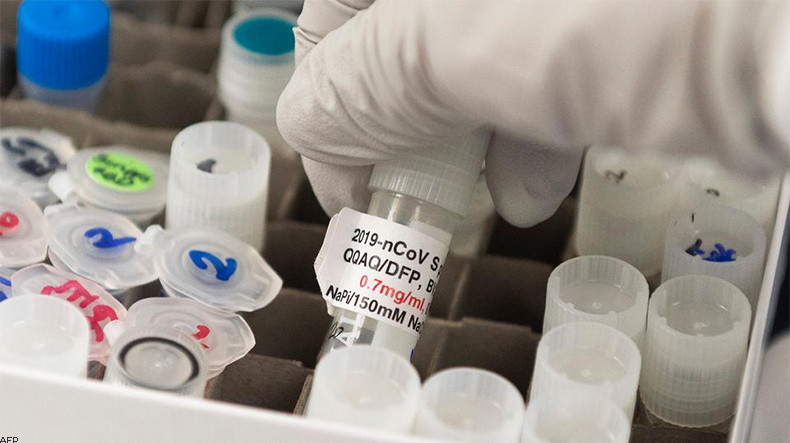
Novavax will inject 131 volunteers in the first phase of the trial testing the safety of the vaccine and looking for signs of its effectiveness, the company’s research chief Dr. Gregory Glenn said.
About a dozen experimental vaccines against the coronavirus are in early stages of testing or poised to start, mostly in China, the U.S. and Europe. It’s not clear that any will prove safe and effective. But many work in different ways, and are made with different technologies, increasing the odds that at least one approach might succeed.
“We are in parallel making doses, making vaccine in anticipation that we’ll be able to show it’s working and be able to start deploying it by the end of this year,” Glenn told a virtual news conference in Melbourne from Novavax’ headquarters in Maryland.
Animal testing suggested the vaccine is effective in low doses. Novavax could manufacture at least 100 million doses this year and 1.5 billion in 2021, he said.
Manufacture of the vaccine, named NVX-CoV2373, was being scaled up with $388 million invested by Norway-based Coalition for Epidemic Preparedness Innovations since March, Glenn said.
The results of the first phase of clinical trials in Melbourne and Brisbane are expected to be known in July, Novavax said. Thousands of candidates in several countries would then become involved in a second phase.
Related news
- Inovio says COVID-19 vaccine produces antibodies in mice, guinea pigs
- Coronavirus vaccine trial by Noubar Afeyan's company shows promising results
- White House health advisor comments on coronavirus vaccine by Noubar Afeyan's company
Newsfeed
Videos