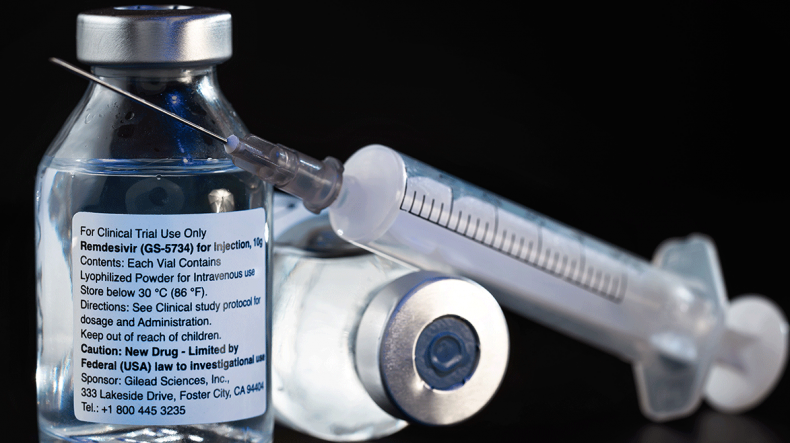
EU grants conditional clearance to COVID-19 antiviral remdesivir
The European Commission said on Friday it had given conditional approval for the use of COVID-19 antiviral remdesivir following an accelerated review process, Reuters reported.
The EU executive said the drug, produced by Gilead Sciences Inc, was the first medicine authorised in the European Union for treating COVID-19 following a “rolling review” begun by the European Medicines Agency at the end of April.
The agency reviews data as they become available on a rolling basis, while development is still ongoing.
The Commission said on Wednesday it was in negotiations with Gilead to obtain doses of remdesivir for the 27 European Union countries.
Newsfeed
Videos






























